Innovative Light Sources for Endoscopic Procedures
Author: Bill Grube, Mufid Alfaris
Narrow endoscopic equipment has been used in surgeries targeting small spaces and structures within the body for many decades. Advances in technology are now accelerating the development and adoption in an increasing number of clinical areas, including ophthalmology, neurology, and gastroenterology. The ability to deliver illumination, imaging, and surgical functionality through sub-millimeter-diameter endoscopes is enabling increasingly-complex and beneficial procedures, while minimizing the disruption to healthy tissues.
Key Advantages of Energetiq's LDLS™
- Broad wavelength range
- High radiance (brightness)
- High spatial stability
- High long-term stability
Evolution of Endoscopy in Ophthalmic Surgery
The first use of an endoscope during ophthalmic surgery was reported in 1934, when Thorpe removed an intraocular foreign body using integrated forceps [1,3]. In 1978, Norris and Cleasby described a 1.7mm diameter (between 13 and 14 gauge) intraocular endoscope for use in surgery [1,4]. By 1990, a 0.6 mm diameter (20-gauge) endoscope was used to provide imaging on a video monitor [2,5,6]. This was soon followed by the first instances of laser surgery performed using endoscopes [2,7,8].
Endoscopy offers unique views of the interior structures of the eye and can be used for a variety of anterior and posterior segment surgeries. Endoscopy enables visualization of structures that are not otherwise accessible. Endoscopes are now available for visualization and, in some cases, include integrated lasers for incisions and biopsies.
Other Sub-millimeter Endoscopy Applications
In addition to ophthalmic surgery, sub-millimeter endoscopy is being applied to a variety of medical procedures aimed at performing minimally- invasive biopsies in organs such as the pancreas,[9] and imaging difficult-to-access spaces in the body, including deep regions of the brain [10]. Narrow endoscopes are also used in industrial settings for remote visual inspection.
Innovative Light Sources for Endoscopy
Energetiq’s unique Laser-Driven Light Source (LDLS™) technology uses a continuous wave laser to couple energy into a plasma in a high- pressure xenon bulb. The resulting plasma is smaller, brighter, and offers higher spatial stability compared to traditional arc lamp sources. The LDLS plasma approximates a point source and provides an advantage when collimating the output. The high spectral radiance and spatial stability of the plasma result in higher efficiency when coupling into a small aperture or fiber. Figure 1 demonstrates coupling of light from two LDLS models (EQ-99X-FC and EQ-77) into an example fiber with a narrow core diameter of 200µm and a small numerical aperture of 0.12.
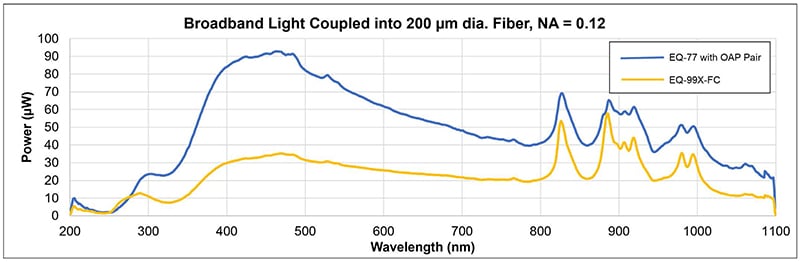
Figure 1: EQ-99X-FC and EQ-77 Laser-Driven Light Sources coupling light into 200 µm diameter, NA=0.12 fiber.
The internal diameter of a sub-millimeter endoscope ranges from roughly 400 µm to 700 µm. For surgical procedures, this space is sometimes shared between separate optical fibers for illumination, imaging, and laser light delivery. Depending on how the endoscope is segmented, the illumination fiber, or fiber bundle, might have a cross-sectional area equivalent to a circle with a diameter of between 200-500 µm. For some applications, a fiber with a small numerical aperture is also desirable. The high spectral radiance and spatial stability of the LDLS enable more light to be coupled into a fiber with a small core diameter and/or small numerical aperture.
The EQ-99X-FC is a fiber-coupled LDLS and the EQ-77 is a higher-power LDLS that is paired with a two off-axis parabolic mirrors to collimate and then focus the light into the narrow fiber. Despite the small fiber diameter and numerical aperture, the EQ-99X-FC and EQ-77 couple approximately 2 and 5 Lumens of visible light into the fiber respectively.
Conclusion
The rapidly-expanding number of applications for sub-millimeter endoscopes are enabled by the ability to couple broadband light into fibers with small core diameters and numerical apertures. The broadband output, small plasma size, high radiance, and high spatial stability of the Laser-Driven Light Source can offer unique benefits for these applications.
References
- Endoscopy-assisted vitrectomy in the anterior vitreous Yong-Zhen Yu, Yu-Ping Zou, Xiu-Lan Zou; 2018
- Retina Today, The Role of Endoscopy in Vitreoretinal Surgery Today; Current technology brings advantages along with some tradeoffs. Jorge G. Arroyo, MD, MPH, January 2012
- Thorpe HE. Ocular endoscope: instrument for removal of intravitreous non-magnetic foreign bodies. Trans AM Acad Ophthalmol Otolaryngol 1934:39:422-424
- Norris JL, Cleasby GW. Intraocular foreign body removal by endoscopy. Ann Ophthalmol 1982; 14(4): 371-372.
- Volkov VV, Danilov AV, Vassin LN, Frolov YA. Flexible endoscope for intraocular surgery. Arch Ophthalmol. 1990;108(7):1037-1038.
- Eguchi S, Araie M. A new ophthalmic electronic videoendoscope system for intraocular surgery. Arch Ophthalmol. 1990;108(12):1778-1781.
- Uram M. Ophthalmic laser microendoscope ciliary process ablation in the management of neovascular glaucoma. Ophthalmology. 1992;99(12):1823-1828.
- Uram M. Ophthalmic laser microendoscope endophotocoagulation. Ophthalmology. 1992;99(12):1829-1832.
- Light scattering spectroscopy identifies the malignant potential of pancreatic cysts during endoscopy, Lei Zhang1†,
Douglas K. Pleskow2†, Vladimir Turzhitsky1, Eric U. Yee3, Tyler M. Berzin2, Mandeep Sawhney2, Shweta Shinagare3, Edward Vitkin1, Yuri Zakharov1, Umar Khan1, Fen Wang2, Jeffrey D. Goldsmith3, Saveli Goldberg4, Ram Chuttani2, Irving Itzkan1, Le Qiu1* and
Lev T. Perelman1, 2, 5* - A minimally invasive lens-free computational microendoscope, Jaewook Shin1, Dung N. Tran1, Jasper R. Stroud1, Sang Chin1,2,3,4, Trac D. Tran1, Mark A. Foster1.


