Advanced Illumination for Biological Hyperspectral Imaging
Author: S. Gunnell
Hyperspectral imaging techniques allow for spatially and spectrally encoded, pixel-by-pixel collection and processing of information across broad bands of the electromagnetic spectrum. Techniques based on this principle are being applied to an expanding range of fields including, but not limited to astronomy, geoscience, agriculture, and more recently biomedical imaging and molecular biology. Energetiq’s high-brightness broadband Laser-Driven Light Sources (LDLS™) are enabling new insights into the biological sciences, especially in applications where researchers employ hyperspectral imaging to study subcellular structures on the nanoscale.
Key Advantages of LDLS
- Broadband spectral output (170 nm-2500 nm)
- Small plasma size (~100 μm)
- Very high brightness
- Excellent spatial and power stability
Ultraviolet Hyperspectral Interferometric Microscopy
Researchers at the Georgia Institute of Technology used UV light from Energetiq’s EQ-99X LDLS™ to illuminate live red blood cells and human neutrophils in a new technique they are calling Ultraviolet Hyperspectral Interferometric Microscopy (UHI). The high-brightness UV output of the LDLS, combined with an interferometric optical set-up, allows for coherent detection of nanometer-scale spatial fluctuations on the sample and highly-sensitive, label-free molecular imaging. [1]
In this particular application, the EQ-99X enables the recovery of sample attenuation, dispersion, and quantitative phase information across a broad spectral band (250-450 nm), where previous studies in the UV region relied on expensive lasers and covered only one or two monochromatic wavelengths. Ultimately, access to a continuous region of the ultraviolet spectrum with brightness orders of magnitude higher than a typical deuterium lamp allows researchers to leverage the specificity of UV spectroscopy for high-resolution molecular imaging, opening up the potential for new insights into how our bodies work on the nanoscale. [1]
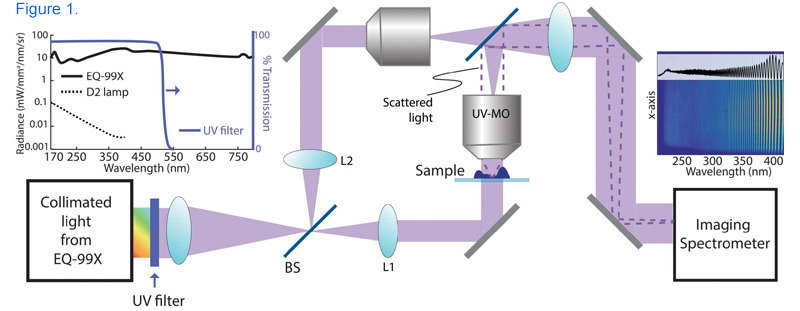
Schematic of the UHI microscopy setup where the collimated broadband beam (radiance spectrum of the source output light is depicted in the left inset). The beam is split into reference and sample beams via the first beam splitter (BS) and collimated using L1 and L2 lenses. The imaging is performed using a UV microscope objective (UV-MO) and the interferometric data (a sample image is shown in the right inset) is recorded by the imaging spectrometer. [1]
Nanoscale Nuclear Architecture Mapping for Cancer Detection (nanoNAM)
In a different technique, researchers at the University of Pittsburgh used the EQ-99X to illuminate cells with light from 480-700 nm in order to map their optical density properties. An increase in optical density caused by nanoscale changes architecture has proven to be a useful indicator for cancer in cells that appear normal when viewed under traditional imaging modalities. The 250μm field of view is simultaneously illuminated by a broadband reference beam and monochromatic light generated by coupling the EQ-99X to an acousto-optical tunable filter. [2]
For each of the >200 wavelengths, a detector records a spectral interference signal between backscattered waves from within the tissue sample and the reference waves. This spatially and spectrally encoded data cube is then used to generate a map of optical density across the cell, which can be further analyzed quantitatively to detect cancer. When combined with co-registered bright field and quantitative phase images also illuminated by the EQ-99X, nanoNAM becomes a powerful tool for label-free imaging with significant clinical potential. [2]
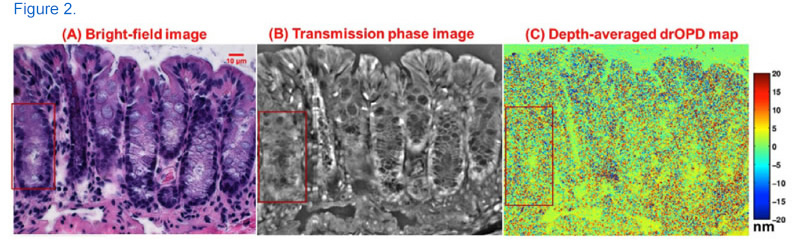
Above: Nuclear architecture maps obtained from the three imaging modalities of nanoNAM optical microscopy system: (A) Bright-field image of an H&E-stained colon tissue; (B) corresponding transmission quantitative phase image; (C) depth-averaged drOPD maps from an unstained colon tissue section.
Sources
1. Ultraviolet Hyperspectral Interferometric Microscopy. A Ojaghi, ME Fay, WA Lam, FE Robles. Scientific Reports (2018) - nature.com, https://doi.org/10.1038/s41598-018-28208-0
2. Fourier phase based depth-resolved nanoscale nuclear architecture mapping for cancer detection. S. Uttam, Y. Liu, Methods (2017), https://doi.org/10.1016/j.ymeth.2017.10.011
Note: This page was originally published in 2018 and was updated in May 2021.


